InSilicoMinds specializes in providing AI and advanced modeling and simulation solutions for Pharma & Life Sciences. We leverage the best of breed validated global solutions and service providers to offer the best in class in silico solutions which help our clients optimize their drug development cycle, reduce costs, save time, and improve patient outcomes. InSilicoMinds enhances its portfolio of computational solutions through an exclusive partnership with InSilicoTrials, a premier provider of AI and simulation tools for drug development, reflecting both companies’ commitment to innovation and excellence in advancing drug research and development.
We are a team of experienced life sciences, digital innovation, and in silico experts that work together to provide our in silico solution. Our team has extensive experience working with a wide range of pharmaceutical and life sciences clients, from small startups to large multinational corporations. We understand the unique challenges facing these industries, and we have the knowledge and expertise to help our clients succeed.
Expertise

Our AI, Modeling & Simulation experts work closely with our clients to understand their unique scientific questions of interest and challenges. We then leverage our expertise in modeling and simulation to provide innovative solutions and insights.
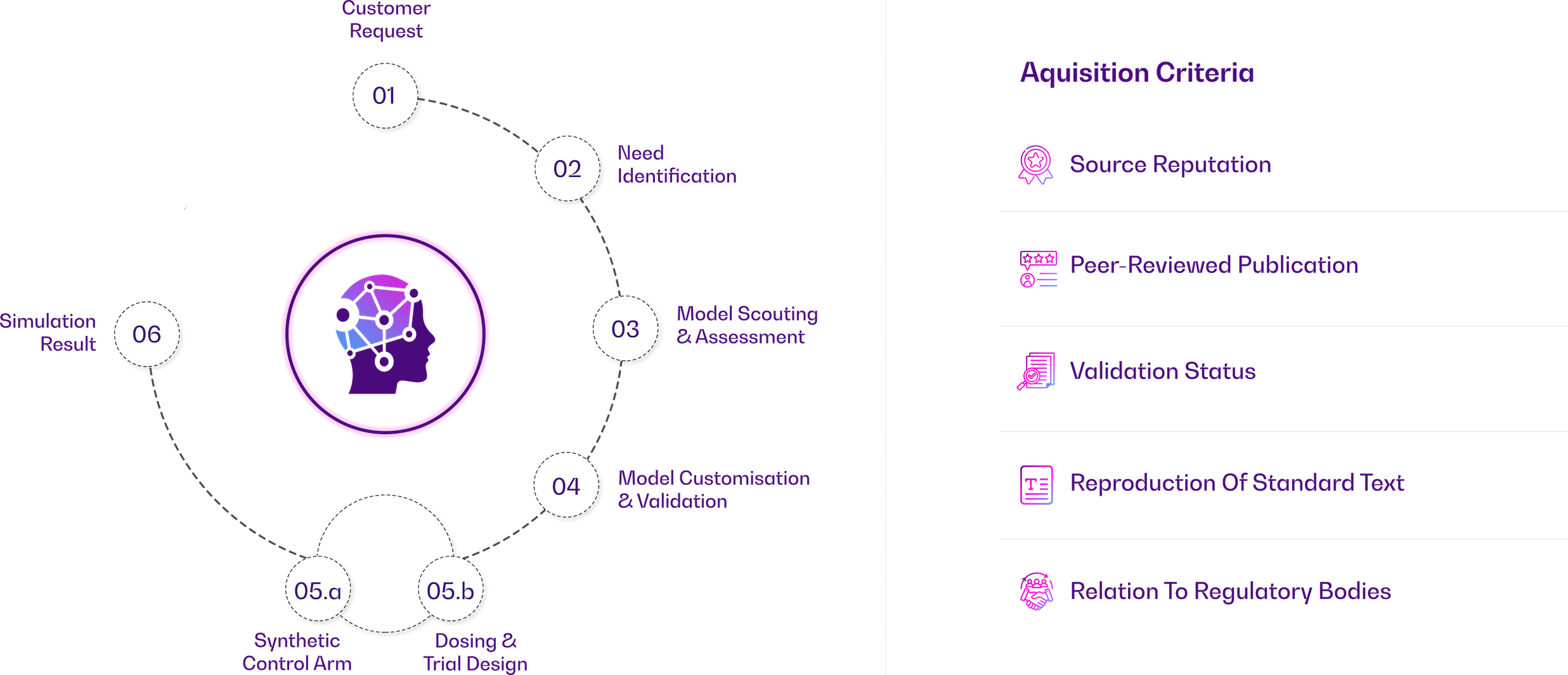
Our AI, Modeling & Simulation experts leverage a combination of best-of-breed software and proprietary in-house tools to deliver comprehensive solutions in the field of AI, modeling, and simulation.




































MODELING AND SIMULATION is a proven scientific approach used to inform crucial drug development decisions. Accepted by regulators and used across the development cycle, it integrates knowledge and relationships between the drug product, safety & efficacy, disease, patient populations and clinical trial parameters.
GxP are a collection of good practices and in the Biomedical field we have Good Laboratory (GLP), Good Clinical Practice (GCP), Good Manufacturing Practices (GMP)…and now Good Simulation Practice (GSP)
An international consortium of over 144 experts from academia, industry, and regulators—including the Avicenna Alliance, VPH Institute, the EU-funded project InSilicoWorld, and 13 M&S experts from FDA’s ModSimWG—are collaborating to establish consensus on best practices for computer modeling and simulation in regulated environments. Their collective work, “Toward Good Simulation Practice,” will be published as an open-access book by Nature Springer with Luca Emili (InSilicoTrials) and Marco Viceconti (University of Bologna) as the editors.
Best Practices for the Use of Computational Modelling and Simulation in the Regulatory Process of Biomedical Products
Editors: Marco Viceconti, Luca Emili

MODELING AND SIMULATION is a proven scientific approach used to inform crucial drug development decisions. Accepted by regulators and used across the development cycle, it integrates knowledge and relationships between the drug product, safety & efficacy, disease, patient populations and clinical trial parameters.

InSilicoMinds enhances its portfolio of computational solutions through an exclusive partnership with InSilicoTrials, a premier provider of AI and simulation tools for drug development, reflecting both companies’ commitment to innovation and excellence in advancing drug research and development.

InSilicoMinds expands its influence in accelerating medicine as an esteemed Avicenna Alliance. This strategic collaboration unites key healthcare stakeholders committed to establishing in silico practices as the norm, ultimately benefiting the pharmaceutical industry and patients globally.

Our company is the best in the industry due to several differentiators that set us apart from our competitors. First and foremost, we have a strong focus on collaboration and scientific research, with over 70 partnerships in this area with regulatory agencies, academic research institutes, and healthcare organizations worldwide.
Additionally, we have access to an impressive $300+ million worth of disease models, in silico tools, and other resources that allow us to stay at the forefront of our field.
Our extensive domain experience is another key advantage, as we have worked with all types of biomedical and pharmaceutical products, including LAIs, biosimilars, vaccines, proteins/peptides, large and small molecules, NCEs/NBEs, and more.
Our validated disease models across various therapeutic areas, such as oncology and neurology, further demonstrate our commitment to providing a breadth and depth of solutions for pharmaceutical development. Finally, we are proud to be GSP compliant, which ensures that our products and services meet the highest standards of safety and quality.
We are a team of experienced life sciences, digital innovation, and in silico experts that work together to provide our in silico solutions.

Nirnith Devireddy
Co-founder & CEO
The Founder and CEO of InSilicoMinds (Ikimind Pvt Ltd.). Nirnith is a seasoned tech entrepreneur with over a decade of experience. He also Co-founded Anipanion, the world’s leading veterinary virtual care platform.
He has completed his Executive Education at Harvard Medical School in Implementing AI Solutions in Healthcare & has a BBA from Babson College with a concentration in Technology, Entrepreneurship, & Design.

Dr. Pranav Karmwar
Chief Scientific Officer
Dr. Pranav is a seasoned AI, Modeling & Simulation professional in the pharmaceutical industry with over a decade of experience in various MNCs. His expertise lies in biopharmaceutics control strategies and modeling and simulation activities for the development of R&D projects. Pranav led the development and optimization of biopharmaceutical products using modeling and simulation techniques, ensuring project goals are met on time and within budget. He has collaborated with cross-functional teams to develop and implement successful product development strategies and overseen the submission of regulatory documents to ensure compliance with applicable regulations and guidelines.

Mytreyi Kuncham
Senior Program Director
A dedicated Recruitment & Business Development Expert specializing in the Health & Life Sciences industry, with a track record of connecting exceptional talent with leading organizations. She holds a Masters Degree in Business Administration specializing in HR and Marketing. Known for developing effective recruitment strategies, nurturing candidate relationships, and providing valuable industry insights. Experienced in developing businesses, client engagement, team leadership, and data-driven decision-making. Passionate about advancing healthcare and life sciences through talent acquisition, HR & business development.

Ayush Rahangdale
Research Scientist
A biopharmaceutics modeling and simulation specialist with expertise in advanced modeling techniques for drug development and data-driven solutions in healthcare and life sciences. He holds a Master’s degree in Bioinformatics, excel in Python programming, machine learning, deep learning, and statistical modeling, and have hands-on experience with bioinformatics/computational biology tools. During his tenure at Novo Nordisk, he contributed to projects that utilized clinical and non-clinical data for drug product development analysis. He also worked on projects for metabolomics biomarker identification, PK/PD, PBPK, and IVIVC modeling.

Dr. Shweta Singh Chauhan
Senior Scientist
Dr. Shweta, an accomplished expert in the field of pharmaceutical sciences, brings a wealth of knowledge and experience in utilizing modeling and simulation tools to facilitate the synthesis of APIs (Active Pharmaceutical Ingredients). With a strong background in molecular modeling, molecular dynamics simulation, and single and multiple ligand simultaneous docking for structure-based drug discovery, she has made significant contributions to the advancement of pharmaceutical research.
Her expertise extends to the development of QSAR models and in-silico based web platforms for the prediction and identification of novel molecules and drugs, as well as the application of computational biology and cheminformatics approaches for toxicity endpoint prediction, chemical screening, interpretation, exploration of chemical space, and biomarker identification.
Our advisory board brings together a wealth of experience from the Pharma and IT sectors, with decades of industry knowledge to guide our decision-making.

Dr. Uday Saxena
Ex-CSO of Dr.Reddy’s / Co-Founder of ReaGene Biosciences
A seasoned Pharma executive with over 30 years of leadership experience.
Dr. Uday Saxena is currently a Co-Founder of start-up Biotech Company , ReaGene Innovations. He has held Executive and Leadership positions at Parke-Davisin Ann Arbor, AtheroGenics in Atlanta as VP of Drug Discovery, Dr.Reddy’s Laboratories as Chief Scientific Officer, (US and India) and Kareus Therapeutics as CEO.
He has a PhD in Biochemistry from Memorial University and Post-doctoral training at Columbia University. He was associated with the Team at Parke-Davis/Pfizer that discovered Lipitor/atorvastatin, the largest selling(peak sales of $17 billion dollars) drug in pharma business. He led teams that brought several drug candidates from ideation to clinic
Dr.Saxena is also an executive counci member of Federation of Asian Biotechs Associations (FABA) and actively participates in the growth of the pharmaceutical sector in India.

Rama Lingireddy
IT, Education and Pharma Entrepreneur
Ramakrishna Lingireddy (Ram) is an engineering graduate from Nagarjuna University, worked in IT industry for about 25 years. He has worked for companies like IBM, British Telecom and Capgemini. In Capgemini, where he worked for 16 years he was the Vice President and Location Head for Hyderabad managing a team of 30000 employees.
In his professional role he worked on enterprise cloud integration & BPM technologies and managed a portfolio of about $0.5 Bn
He travelled extensively across the globe and spent 9 years in the UK managing various large customer engagements for global MNCs. He has been an active investor in a few start-ups ( 3D printing company, Digital Healthcare and AI In Biopharma) where he has been providing the mentorship.
As a part of his entrepreneurial journey, Ram has joined a business group which is spread across IT, Education, Power and Pharma sectors set-up under the guidance of promoter of the 2nd largest Pharma company in India. He has been associated with Hysea leadership team for last 3 years and currently serving as General Secretary

Dr. G Satyanarayana Sinha
Strategic Advisor
A seasoned Pharmaceutical executive with over 35 years of experience in the Quality Systems of Pharmaceuticals Manufacturing, Testing and Logistics.
Mr. Sinha has Worked in various organizations of repute such as Proctor & Gamble, Wander Limited, Ricon Pharma and Vista Pharma and various departments in the Government of India such as the Directorate General of Health Services and Directorate General of Quality Assurance.

Srinath Devireddy
IT Entrepreneur
A dynamic executive who leverages solid leadership skills to achieve success and drive competitive advantage. Proficient in all aspects of global delivery models for IT and BPO Services. Srinath is an IT-entrepreneur and runs Adroitent, a business intelligence, quality assurance and healthcare focused IT solutions company. He is an angel investor for GenY Medium, Ekincare, Anipanion and other startups.
He has held several CXO positions in the IT industry prior to starting Adroitent. He has extensive experience in the travel domain. He rose up the ranks to become Vice President of Operations at Travelocity in-charge of operations management and technology. Post Travelocity, he headed global operations for Patni Computers. He was President of TRX Asia, a travel technology solutions company.

Dr. Sai Phanindra Venkatapurapu (PhD)
Strategic Advisor
Sai is a Computational Biologist with a passion to develop and apply novel computational methods and digital tools to solve problems in the healthcare industry. He is experienced in developing digital tools powered by mechanistic mathematical models, advanced data analytics, and machine learning algorithms to acclerate drug development and better manage individual and population health. Sai has over a decade of post-PhD experience in modeling and simulation, digital health product development and management, operations and strategy consulting focused on improving patient outcomes. Currently, Sai is a Senior Manager at PwC, where he leads a team of biomedical scientists and engineers to develop and maintain a proprietary simulation modeling platform. He also builds simulation model-based digital products powered by digital twins to help patients, payors and pharmaceutical companies manage chronic diseases and reduce overall healthcare costs. Prior to joining PwC, Sai worked as a Mathematical Modeler at Immunetrics. He has a PhD in Bioinformatics and Computational Biology from the University of North Carolina at Chapel Hill and a Bachelors in Biotechnology from the Indian Institute of Technology Guwahati. Sai also serves as an adjunct faculty at Rishihood University in Sonipat.
Connect with our in silico team of experts to discuss AI solutions and advanced modeling and simulation for the Pharma and Life Science industries and hyper accelerate your success
Our Locations
Copyrights 2024 Ikiminds Pvt Ltd. All Rights Reserved