

InSilicoMinds specializes in providing AI and advanced modeling and simulation solutions for Pharma & Life Sciences. We leverage the best of breed validated global solutions and service providers to offer the best in class in silico solutions which help our clients optimize their drug development cycle, reduce costs, save time, and improve patient outcomes. InSilicoMinds enhances its portfolio of computational solutions through an exclusive partnership with InSilicoTrials, a premier provider of AI and simulation tools for drug development, reflecting both companies’ commitment to innovation and excellence in advancing drug research and development.
We are a team of experienced life sciences, digital innovation, and in silico experts that work together to provide our in silico solution. Our team has extensive experience working with a wide range of pharmaceutical and life sciences clients, from small startups to large multinational corporations. We understand the unique challenges facing these industries, and we have the knowledge and expertise to help our clients succeed.
Expertise

The development of generic drugs is a complex and costly process that requires a significant investment of time and resources. Our approaches involve the use of computational methods to simulate and predict the behavior of drugs in the body, optimize drug formulations and predict potential safety issues for various dosage forms such as Solid orals, Complex injectables, Transdermal patches etc.
The development of vaccines is a critical component of global health efforts, but it is a complex and time-consuming process. Our computational tools can play a vital role in streamlining and de-risking vaccine development to achieve a faster process, lower costs, and higher vaccine efficacy.
The life science industry has long faced the lengthy and costly process of developing new drugs and medical devices. Digital Twin and Biosimulation are the key to cutting down time frames and costs in this area. Our digital simulations help companies reduce by up to 50% the time-consuming and costly development, as well as subsequent registration / certification processes of new drugs and medical devices.
Conventional API production processes are time-consuming and costly due to intricate chemical reactions, extensive purification, rigorous quality control, and specialized requirements. Our AI and in silico solutions improve API production efficiency by leveraging computational methods to streamline drug discovery, accurately predict reaction outcomes, and offer comprehensive assistance in optimizing synthesis processes. By utilizing our expertise and tools, pharmaceutical developers can expedite the development of safe and effective APIs for commercialization.

Our company is the best in the industry due to several differentiators that set us apart from our competitors. First and foremost, we have a strong focus on collaboration and scientific research, with over 70 partnerships in this area with regulatory agencies, academic research institutes, and healthcare organizations worldwide.
Additionally, we have access to an impressive $300+ million worth of disease models, in silico tools, and other resources that allow us to stay at the forefront of our field.
Our extensive domain experience is another key advantage, as we have worked with all types of biomedical and pharmaceutical products, including LAIs, biosimilars, vaccines, proteins/peptides, large and small molecules, NCEs/NBEs, and more.
Our validated disease models across various therapeutic areas, such as oncology and neurology, further demonstrate our commitment to providing a breadth and depth of solutions for pharmaceutical development. Finally, we are proud to be GSP compliant, which ensures that our products and services meet the highest standards of safety and quality.
Our AI, Modeling & Simulation experts work closely with our clients to understand their unique scientific questions of interest and challenges. We then leverage our expertise in modeling and simulation to provide innovative solutions and insights.
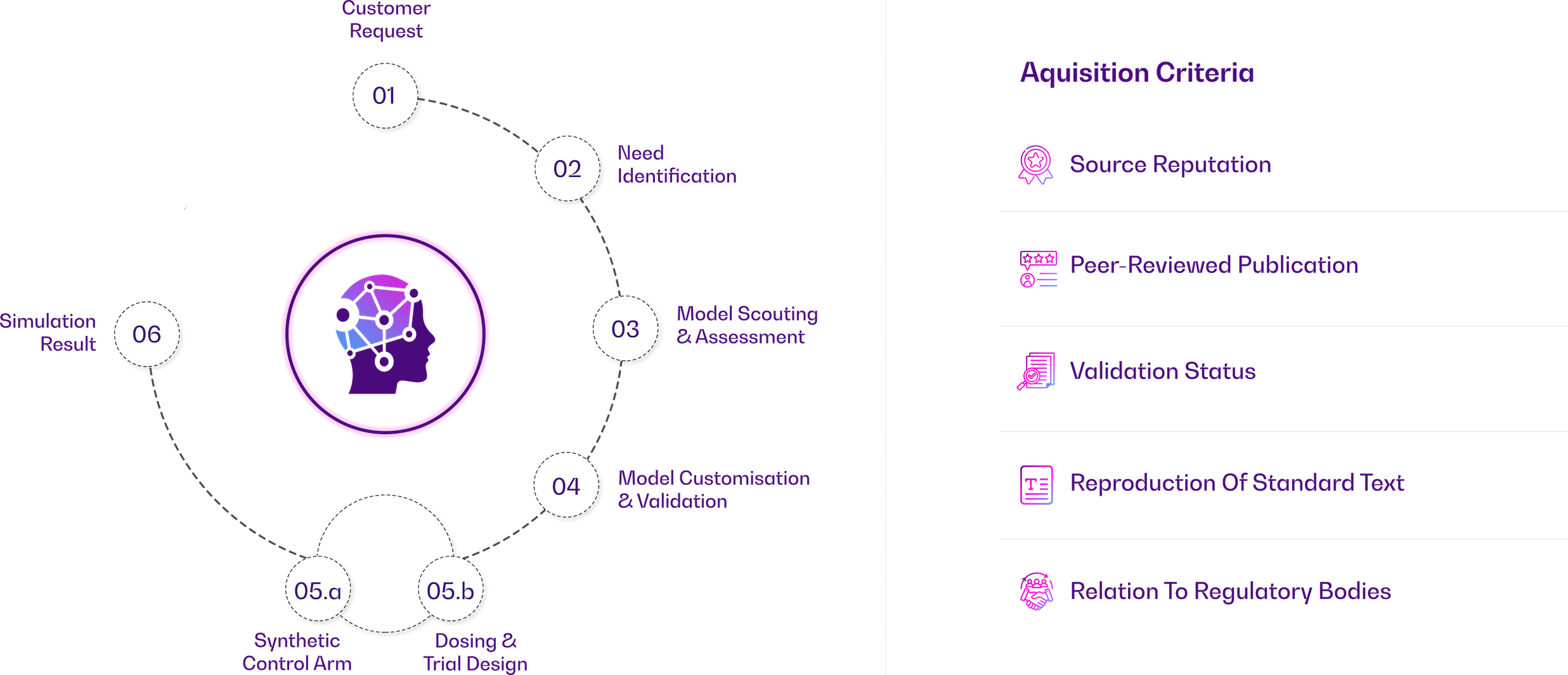
Our AI, Modeling & Simulation experts leverage a combination of best-of-breed software and proprietary in-house tools to deliver comprehensive solutions in the field of AI, modeling, and simulation.




































MODELING AND SIMULATION is a proven scientific approach used to inform crucial drug development decisions. Accepted by regulators and used across the development cycle, it integrates knowledge and relationships between the drug product, safety & efficacy, disease, patient populations and clinical trial parameters.
GxP are a collection of good practices and in the Biomedical field we have Good Laboratory (GLP), Good Clinical Practice (GCP), Good Manufacturing Practices (GMP)…and now Good Simulation Practice (GSP)
An international consortium of over 144 experts from academia, industry, and regulators—including the Avicenna Alliance, VPH Institute, the EU-funded project InSilicoWorld, and 13 M&S experts from FDA’s ModSimWG—are collaborating to establish consensus on best practices for computer modeling and simulation in regulated environments. Their collective work, “Toward Good Simulation Practice,” will be published as an open-access book by Nature Springer with Luca Emili (InSilicoTrials) and Marco Viceconti (University of Bologna) as the editors.
Best Practices for the Use of Computational Modelling and Simulation in the Regulatory Process of Biomedical Products
Editors: Marco Viceconti, Luca Emili

MODELING AND SIMULATION is a proven scientific approach used to inform crucial drug development decisions. Accepted by regulators and used across the development cycle, it integrates knowledge and relationships between the drug product, safety & efficacy, disease, patient populations and clinical trial parameters.

InSilicoMinds enhances its portfolio of computational solutions through an exclusive partnership with InSilicoTrials, a premier provider of AI and simulation tools for drug development, reflecting both companies’ commitment to innovation and excellence in advancing drug research and development.

InSilicoMinds expands its influence in accelerating medicine as an esteemed Avicenna Alliance. This strategic collaboration unites key healthcare stakeholders committed to establishing in silico practices as the norm, ultimately benefiting the pharmaceutical industry and patients globally.
Connect with our in silico team of experts to discuss AI solutions and advanced modeling and simulation for the Pharma and Life Science industries and hyper accelerate your success
Our Locations
Copyrights 2024 Ikiminds Pvt Ltd. All Rights Reserved